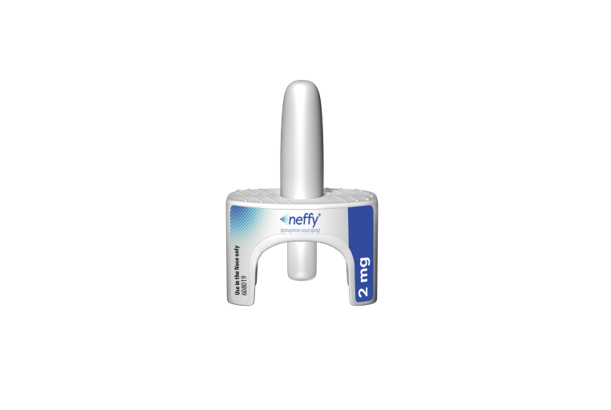
Crystal Lake, Illinois, August 15, 2024 – AptarGroup, Inc. (NYSE: ATR), a global leader in drug and consumer product dosing, dispensing and protection technologies, today announced that its Unidose Liquid System (Unidose) is the delivery system approved with neffy® (epinephrine nasal spray), the first and only needle-free treatment approved by the U.S. FDA for the emergency treatment of patients with allergic reactions (Type I), including anaphylaxis. This marks the first regulatory approval worldwide for nasally-delivered epinephrine.
Aptar’s Unidose is a single-use, ready-to-use, one-step nasal delivery system used to administer neffy to a patient during a severe allergic reaction. During such an event, the patient, healthcare professional, caregiver or user presses a small plunger on the bottom of the nasal spray system to release the drug in a single spray into the nostril.
Stephan B. Tanda, Aptar President and CEO, stated, “Aptar has been a leader in nasal delivery of medication for more than 30 years. We are proud of our role in the pharma industry to increase the use of nasally delivered medications that help promote adherence and ease of use for patients.”
Unidose (UDS) and Bidose (BDS) Technology Platforms
Aptar’s UDS and BDS technology platforms are designed to be robust, reliable and intuitive systems for easy administration by patients or caretakers. These drug delivery systems are designed and manufactured with strict quality controls intended to meet FDA’s guidelines for reliability. They offer biotech and pharmaceutical companies effective and reliable single or two-shot intranasal delivery for a variety of medicines including for emergency use and treatments of severe conditions. They can also be integrated with wireless connectivity technologies.
Accelerated Development Support via Aptar Pharma Services
This novel treatment for severe allergic reaction is an example of a Combination Product submission, and benefited from Aptar Pharma’s Services offering, a comprehensive portfolio of stage-specific development packages. Aptar’s dedicated Regulatory Affairs experts and analytical scientists help customers proactively address regulatory needs to help accelerate approval.
“The approval of neffy, which uses our Unidose System, and is the first nasally-delivered epinephrine treatment for severe allergic reaction, including anaphylaxis, once again demonstrates Aptar Pharma’s ‘formulation to patient’ focus on helping our customers develop complex, innovative treatments,” stated Gael Touya, President, Aptar Pharma. “When we combine our nasal systems’ capabilities with our Aptar Pharma Services offering, we bring added value to our customers, and aim to provide further convenience for patients and their caregivers worldwide.”
Download the full Press Release.