CPS Technology Platform
Multidose Systems, Spray Pumps
Best-in-Class Preservative-Free Nasal Drug Delivery
Superior spray pump supporting for nasal drug application
Aptar Pharma’s CPS spray pump has been optimized for nasal drug applications. Whenever a medication containing an active pharmaceutical ingredient (API) is delivered through the nasal route, targeted and controlled application is key. Our CPS nasal spray is specifically designed to enable reliable, exact dosing and targeted medication delivery to the nasal mucosa. Preservative-free is becoming more important in nasal drug delivery formulations. Our CPS nasal spray provides the best-in-class solution for preservative-free drug formulations, with its proven microbiological integrity, making the need for additives obsolete. Sensitive formulations are protected from oxidation through the metal-free fluid path of CPS’s technology. Any clogging or crystallization on the tip is prevented by the reinforced tip sealing mechanism, making the CPS system a safer solution for nasal drug delivery.
Our versatile CPS Technology Platform is the first choice for non-preserved nasal drug delivery across a wide range of medical applications such as allergic rhinitis, pain, CNS as well as for intra‑nasal vaccination.
Dedicated regulatory support for nasal spray medications
Aptar Pharma has a successful track record of over 150 approved NDAs, ANDAs and INDs in the U.S. in the past 5 years alone.
Our regulatory guidance and support at each stage of your product development facilitates your way to market approval, worldwide.
We have accompanied product approval processes with major regulatory authorities globally and built up regional regulatory expertise. Our experts are familiar with the bundling registration in China and master the requirements of the new Medical Device Regulation in EMEA. Our combination product specialist team specifically supports approval processes of drug combination products according to FDA guidelines.
Based on your regulatory needs and requirements we can tailor make the solution and the support you need.
With over 75 years of experience in nasal spray pump technologies and regulatory expertise, Aptar Pharma is your trusted partner for your next product development.
First choice for preservative-free nasal medications
Preservatives in nasal formulations, such as Benzalkonium Chloride (BAC), can have a negative impact on the ciliated tissue in the nasal cavity. Our CPS is the leading preservative-free spray pump solution for nasal drug applications worldwide.
Our advanced filter membrane prevents the ingress of any microbiological contamination from venting air. This combined with Aptar Pharma’s purely mechanical Tip Seal technology eliminates the need for preservatives and additives, such as silver ions or surface coating.
Microbiological integrity of Aptar Pharma‘s preservative-free multi-dose solutions (PFMS) has been proven by microbial challenge tests, passing relevant worst-case simulations.
Comprehensive Services to accelerate and derisk your drug product development
From our customized drug delivery solutions to our analytical and laboratory testing services, and our elastomer centre providing longstanding know-how optimizing chemical purity for your product development, we offer comprehensive service packages that can ensure an optimized product development process for your specific formulation needs. Analytical laboratory tests and physics tests are provided to optimize our CPS for your specific drug formulation needs. Spray characteristics are optimized for individual formulations according to their specifications. In addition we offer full extractable and toxicology packages to support regulatory filing of your nasal drug formulation.
Our CPS can be specifically customized with the development of individual designs for nasal drug medication products. Multi-disciplinary project teams manage customized product developments in close partnership with you, offering individually designed solutions with the high quality you can expect from Aptar Pharma.
CPS Technology Platform Advantages
Dose volumes of 50 to 140µl can be individually adapted. This provides maximal flexibility for various formulation concentrations and can adapt to specific dosing needs.
CPS nasal spray pumps are characterized through their superior and fast priming, reaching the wanted dose volume quickly. Re-priming is not needed as the dose volume stays stable over time.
The materials used for our CPS nasal spray pumps that are in contact with the formulation are compliant to all main relevant regulatory requirements, such as U.S. and EU Food Contact as well as U.S. and European Pharmacopoeia.
Our CPS can be provided as a 360° spray that enables fully flexible usage. The inner pouch of the container gradually collapses instead of having an inflow of venting air. That way the dip tube is submersed in the formulation to be dispensed, enabling the spraying at any angle.
Our CPS device is US-DMF supported, to help accelerate and derisk your drug product approval by regulatory authorities.
The nasal actuator comes with either a screw-on or snap-on closure, includes a protective cap and can be provided with a safety clip. For nasal serial vaccination purposes, mass vaccination caps can be provided.
Make Your Next Nasal Medication Preservative-Free
To bring your nasal drug delivery formulation to preservative-free levels, contact the Aptar Pharma experts and let us bring our longstanding expertise in nasal spray development to work for you.
We Have a Broad Range of Value Added Services to Accelerate and Derisk
Your Drug Product Development.
Start a Project With Us
We Thrive on Transforming Ideas into Opportunities – Let Yours be Next.
You Might Also Be Interested In These Products
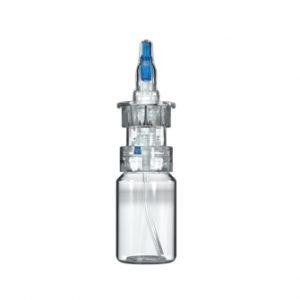
APF Advanced Preservative Free

Bidose (BDS) Liquid Nasal Spray System
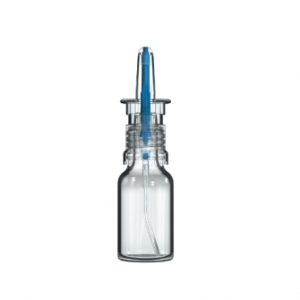
Classic Line and VP6 Technology Platforms



