Request More Information
Closex
Requesting information on Airless+ Dermal Drug Delivery Systems.
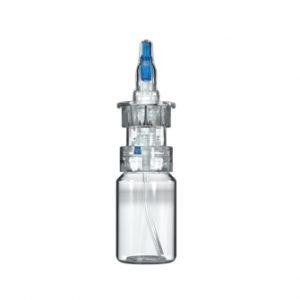
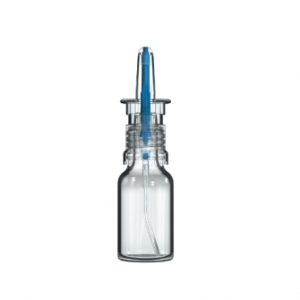
Aptar Pharma’s Airless+ Dermal Drug Delivery systems provide airtight solutions to protect sensitive products across a wide range of formulation viscosities. Utilized in over 50 prescription dermal products launched in regulated markets worldwide, our Airless+ Drug Delivery systems are proven dermal industry leaders.
Offering a comprehensive range of Airless dispensing devices and an extensive number of combinations, including container sizes and actuators, means Aptar Pharma makes it easy to find the right solution for your specific dermal product needs.
Aptar Pharma puts the health and safety of our Airless+ users first by offering Child-Resistant Senior-Friendly (CRSF) safety features with available devices.

Want an exceptional user experience for your dermal products? The advanced design of our Airless+ Dermal Drug Delivery systems supports convenient and intuitive operation. Aptar Pharma’s Airless+ systems were designed to always dispense semi-solid formulations cleanly, safely and reliably. And with 360-degree functionality, they consistently deliver a precise dose at virtually any dispensing orientation.
Our Airless+ Dermal Drug Delivery systems minimize product wastage by efficiently dispensing the maximum amount of formulation from the device. The spent dispenser can then be easily recycled, enhancing the user experience while simplifying sustainability.
Aptar Pharma’s Airless+ systems’ advanced functionality makes them the solution of choice for premium Dermal Drug Delivery brands.
Aptar Pharma’s Airless+ Drug Delivery systems are designed to meet the increasingly strict pharmaceutical regulatory and quality demands for Dermal Drug Delivery systems.
Our Airless+ systems are made with medical grade resins that come with comprehensive change control management, providing transparency to changes in manufacturing processes and materials. We also provide information packages on resin materials and product documentation support for dermal product development and market access. Our Airless+ dermal systems are designed for patient safety and reduced regulatory risks.
Aptar Pharma’s Airless+ Extended Support (ES) is what we call the industry leading service we offer our customers beyond just devices. Extended Support includes a broad range of world-class technical and regulatory services for your dermal products. Our Extended Support is scaled to meet the needs of your individual product development programs. Test packages are designed for a wide range of semi-solid dermal formulation applications. Device extractables data is generated according to USP/ISO requirements, supporting your product’s leachables study. Our Extended Support services save customers both time and money as the critical information supporting our Airless+ systems are already prepared and available to you. Extended Support is designed to help accelerate and derisk your Airless+ Dermal Drug Delivery programs.
Drug-Device Combination product filing requirements are becoming more complex every day. Aptar Pharma’s project and regulatory teams support customers through the latest regulatory filing challenges including US 21 CFR 820.30 or EU MDR 2017/745. Leverage Aptar Pharma’s proven experience in supporting your next Drug-Device Combination product submission. And with Aptar Pharma’s extensive number of combination options, for both visual and functional variations, our Airless+ systems can also help you meet your marketing objectives and increase customer demand.
Working Together Towards A More Sustainable Future With Circularity
Our Airless+ dermal device range supports circularity objectives with recyclable packaging
as well as the use of renewable feedstock for Airless+ devices in a mass-balance approach.
Our Airless+ Dermal Drug Delivery range includes a variety of dispensing systems using medical grade resins for pharmaceutical use. With numerous functional component combinations and the individual piston positioning service performed by Aptar Pharma, we can supply the widest range of Airless+ devices to the pharmaceutical market.
Our flexible Airless+ Dermal Drug Delivery range provides many dosing options ranging from 200µl to 1500µl combined with container size options ranging from 5ml to 200ml. Airless+ dermal devices, with numerous available actuator and closure designs, can easily deliver brand differentiation.
Our self-closing actuators excel at product protection and prevent drying of the next dose of dermal formulation. Aptar Pharma’s Airless+ range includes both Top- and Bottom-Fill models, providing convenient options for your filling requirements.
Our innovative Child-Resistant Senior-Friendly (CRSF) features prevent accidental product access by minors, while being intuitive and easy-to-use for seniors. Our certified child-protection features have become increasingly important in the growing topical pain treatment, medical cannabis/CBD and rosacea care segments for Dermal Drug Delivery products.
Learn more about Child-Resistant Senior-Friendly (CRSF) technology

Aptar Pharma’s service and project support makes packaging development easy. We provide comprehensive services, project and technical support through each step of your dermal product development.
From regulatory affairs expertise and specialized filling support to compatibility and functionality testing services, we can help advance your dermal product to market. In addition, we offer responsive collaboration on product concept development, customized marketing samples and mock-ups to keep your project moving forward.
Aptar Pharma’s regulatory affairs experts are up-to-date with current pharma regulations including those for Drug-Device Combination products. Our project teams offer comprehensive DMF support and data on the well-characterized materials. Airless+ Extended Support services provide you with applicable test reports in submission ready formats.
Aptar Pharma’s Drug-Device Combination product support package includes relevant device documentation meeting 21 CFR 820.30 (US) and MDR 2017/745, GSPR (EU) Drug-Device Combination requirements.


Our Airless+ Dermal Drug Delivery range is fully compliant with current regulatory requirements.
Our support teams can take you from formulation to patient while reducing development risks and increasing speed-to-market.