Intranasal Vaccines
Multidose Systems, Nasal Vaccines
Pharma
With the rapidly expanding demand for vaccines globally, the call for non-invasive alternatives to traditional vaccine injections are growing.
Aptar Pharma offers leading nasal spray technologies that are needle-free, simple to use and can deliver many types of vaccines. Intranasal vaccine delivery is a safe, convenient, and reliable vaccine delivery method.
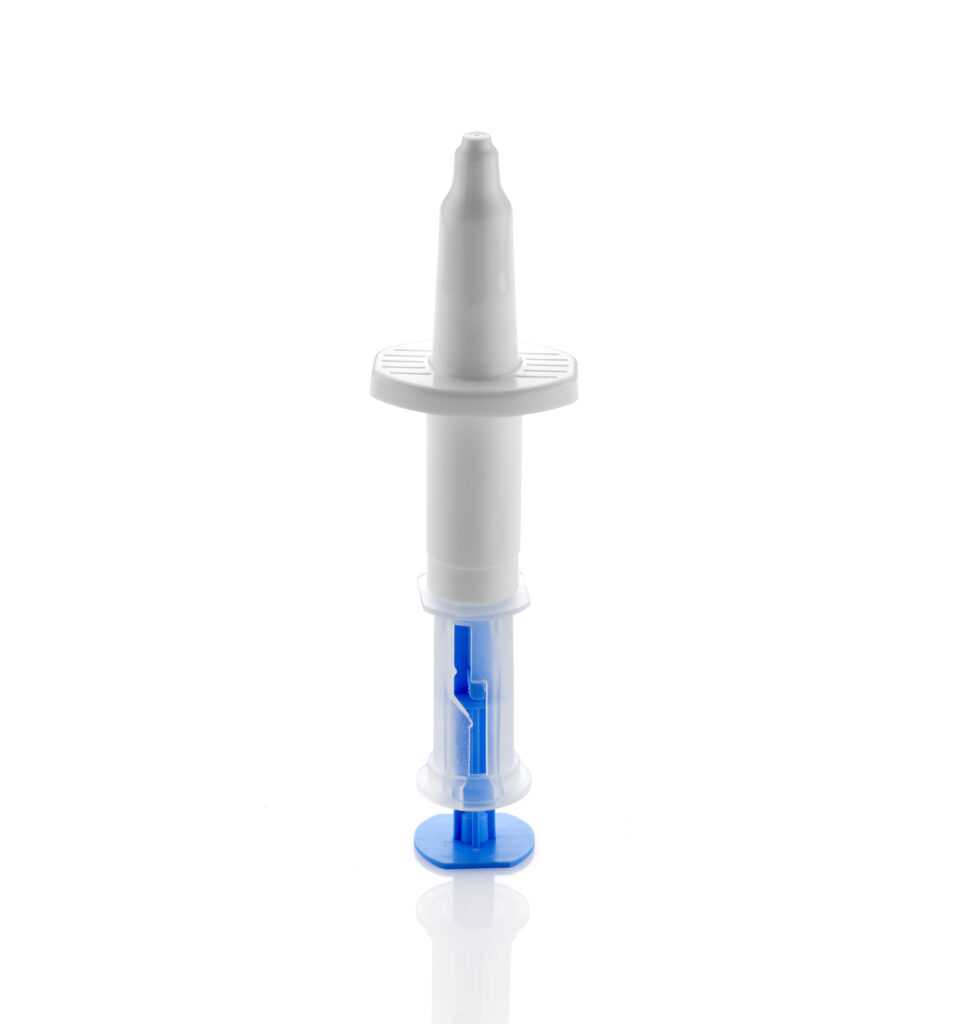
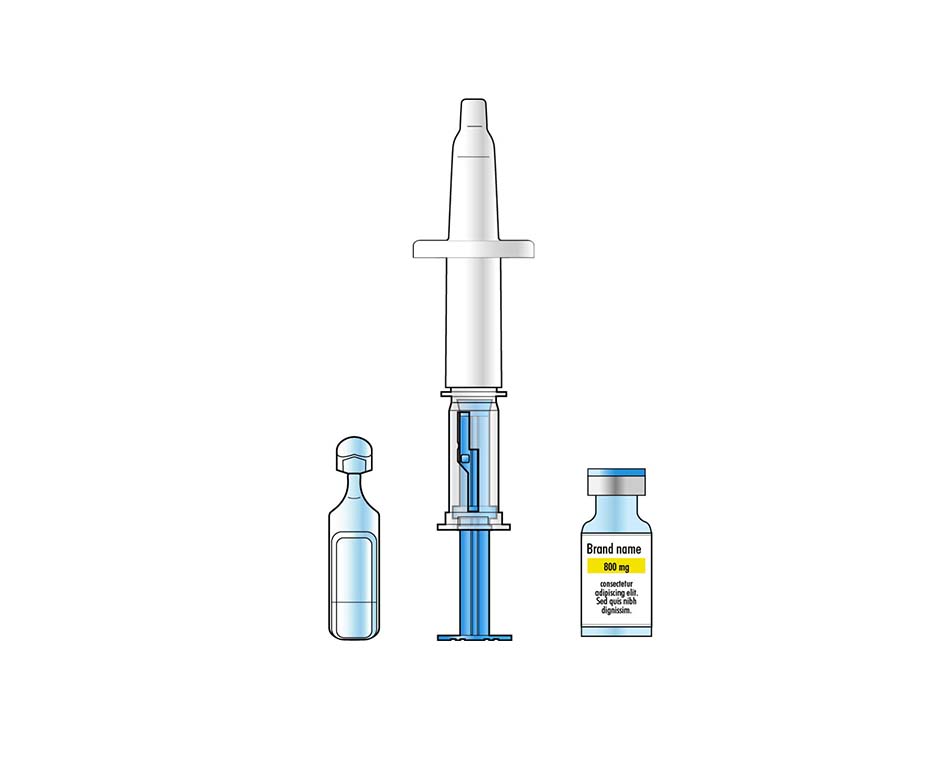




Leading the Way in Intranasal Vaccines
Market Ready
Ideal for today’s vaccine market our intranasal vaccine systems are easy to fill on existing equipment or delivered ready to use by HCP. They are a cost effective, easy to transport, readily scalable and a proven regulatory track record.
Non-invasive & Needle Free
One roadblock to higher levels of patient acceptance of vaccine is fear of painful injections. Nasal vaccines are ideal for children or those with needle fear, as they are a non-invasive and convenient alternative.
Suitable for a Variety of Vaccine Types
With both powder and liquid nasal vaccine options, Aptar Pharma’s nasal vaccine delivery devices are suitable for a wide range of vaccine types including DNA/RNA, live attenuated, inactivated, subunit and viral vectors. Vaccine formulations may contain antigens, adjuvants and a range of available excipients.
Specialized Devices and Features
Aptar Pharma nasal vaccine systems can offer a range of feature driven benefits including variable dose volume opportunities, flexible device platform range, adaptability for liquid, powder and reconstituted formulations.
Accelerate and Derisk with Specialized Support
Aptar Pharma combines its specialized knowledge of intranasal vaccines with the proven success of its advanced nasal vaccine systems. Accelerate vaccine development with the support of our device, formulation, analytical, in-vitro modelling and stability services.
Nasal Vaccine Starter Kit
Aptar Pharma can help you start your new nasal vaccine project by offering a Starter Kit for Intranasal drug delivery.
The kit can also help with antiviral, antibody and immunotherapy projects to help accelerate your new drug development project.
The advantages of this kit are:
- Quick access to a variety of component delivery system at Aptar Pharma for vaccine, antibody and antiviral development programs
- Components included to be able to use the nasal LuerVax delivery system as a transfer device
- Components with spray performance that meet nasal spray guidelines and also deposit in target areas
What’s in the kit?
- 5 Syringes with Needles
- 5 Aptar Pharma LuerVax systems with dose separators (200mcl and 500mcl)
- 5 Aptar Pharma BiVax systems
- 5 Aptar Pharma Bidose Liquid nasal systems

Market Ready
Aptar Pharma’s nasal vaccine systems are ready to meet the needs of the vaccine industry. Our nasal vaccine devices can be filled on a wide range of existing production lines at CMOs or in-house manufacturing facilities or ready to use at the HCP. Production can be quickly scaled up to meet virtually any demand. From early clinical to global commercial scale, nasal vaccines are highly manufacturable. Nasal vaccines can also be cost-effective when compared to traditional sterile injectable vaccines, considering the complexity of multi-component sterile manufacturing.
Some nasal vaccines, including powder vaccines can avoid cold-chain requirement making their transportation to anywhere in the world easier. Offering intranasal vaccines to Health Care Professionals (HCPs) in addition to your existing oral and injectable vaccination offering, enhances your brand differentiation. All these advantages, combined with Aptar Pharma’s proven regulatory success including a U.S. FDA approved market reference, means our nasal vaccine systems are proven market ready for today’s vaccine requirements.

Advantages of Intranasal Vaccination
Development of an effective intranasal vaccine has numerous potential advantages over conventional, subcutaneous or intramuscular-based delivery. First, the nasal passageway is very often the first point of entry for certain pathogens; if the pathogen can be halted in the nasal passages, curtailing the spread of infection further, this could lead to an improved overall prognosis. In addition, the direct immunization of nasal mucosa may promote systemic and mucosal immunity, which may help prevent viral shedding and disease transmission.
Furthermore, self-administration of a nasal powder vaccine provides for “needle free” administration to the patient avoids the need for syringe disposal, and potentially simplifies global distribution with the elimination of extreme cold from the supply chain. These aspects of nasal delivery could open up vaccine availability to larger populations in regions and countries with limited refrigeration infrastructure.
Aptar Pharma’s nasal vaccine systems may be pre-filled to avoid complex or risky on-site preparation steps required for some lyophilized or multicomponent injection systems. Overall, Aptar Pharma’s range of nasal vaccine devices provide an efficient, patient centric solution in both clinical and commercial settings for vaccine administration.

Suitable for a Variety of Vaccine Types
With both powder and liquid nasal vaccine formulation options, Aptar Pharma’s nasal vaccine devices are suitable for a wide range of vaccine types. Nasal sprays present a strong alternative approach for both therapeutic and prophylactic nasal vaccine approaches. Therapeutic treatments of cancer, autoimmune and infectious diseases as well as prophylactic treatments vaccines for Covid-19 have been studied and developed in nasal vaccine formats.
These formulations can incorporate a range of vaccine types including DNA/RNA, live attenuated, inactivated, subunit and viral vectors. Nasal vaccine formulations may contain antigens, adjuvants and a range of available excipients in order to deliver the vaccine to patients intranasally. With such a wide range of possible vaccine types and formulation approaches that are suitable for Aptar Pharma nasal vaccine devices, the number and patient acceptance of nasal vaccines will continue to grow rapidly.
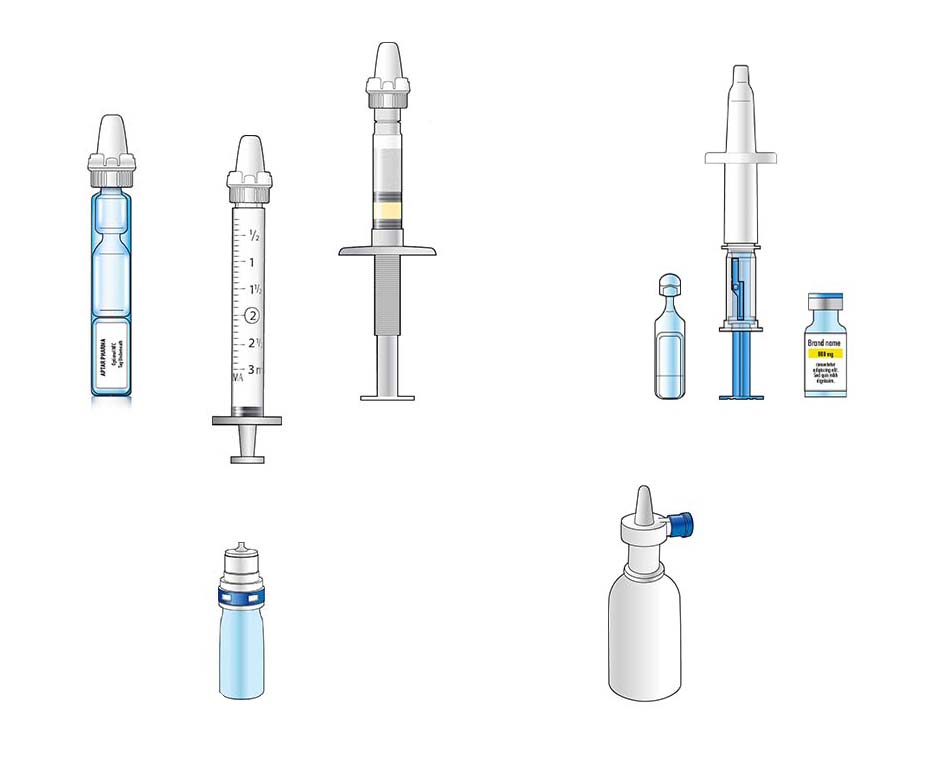
Specialized Devices and Features
Aptar Pharma nasal vaccine systems can offer a range of feature driven benefits, not found with traditional injectable vaccines. Our nasal vaccine devices can handle liquid, powder and reconstituted formulations and offers variable dose volume options through its advanced designs. Sterile device components can provide dosing flexibility and maintain the sterility of the product formulation.
Powder nasal vaccine formulations can utilize minimal formulation, that use very few excipients and exclude moisture, enabling enhanced product stability and extended shelf life. Powder vaccines can also enable high payload delivery when required. The ability to avoid cold chain storage requirements for many powder nasal vaccines, makes global distribution of critical vaccines possible even to remote or undeveloped regions.

Accelerate and derisk with specialized support
With a proven track record as a leader in nasal drug delivery combined with its specialized knowledge of nasal vaccine science, Aptar Pharma is an ideal partner to develop your next nasal vaccine product. Offering advanced nasal vaccine device designs across its range, Aptar Pharma also provides specialized support services to make the development process easy for its customers.
From device selection, formulation/analytical development and regulatory support Aptar Pharma works with you every step of the way. With sophisticated in-vitro modelling and analytical testing, we offer specialized nasal vaccine knowledge and capabilities that you won’t find elsewhere. Our technologies have been utilized in many customer products and combination products that have successfully been approved by leading regulatory bodies including the U.S. FDA and EMA. With numerous market references, Aptar Pharma has the proven regulatory track record to give you confidence in our comprehensive regulatory support services. We take you from formulation to patient.

Partner With Aptar Pharma and
Evolve Your Vaccine Delivery Options
Start you intranasal vaccine project with a world leader in nasal drug delivery.
Check out our Frequently Asked Questions
on Intranasal Vaccination
This Might Also Be of Interest

Overcoming Challenges in Preclinical Studies for Intranasal and Inhalation Programs
Webinars, Pharmaceutical, Innovation & Insights, Drug Delivery Innovations, Market Insights, Product Solutions

Optimising Preclinical Studies for Intranasal and Pulmonary Programmes
Publications, Pharmaceutical, Drug Delivery Innovations, Market Insights, Product Solutions
Targeted Aerosol Delivery to NALT Using BiVax Intranasal Atomizer
Publications, Pharmaceutical, Market Insights, Product Solutions, Drug Delivery Innovations

PureHale® & The Future of Fine Mist Dispensers for Upper Airway Treatments
Publications, Pharmaceutical, Drug Delivery Innovations, Brand Differentiation, Market Insights, Product Solutions
We Offer World-Leading Support Services for You at Every Stage of Your Product Development
Explore How We Serve Your Market
Request More Information
Requesting information on Intranasal Vaccines.
How Can We Help?

