Case Studies, Pharmaceutical
Case Study – Pre-Clinical Respiratory Research with PADA
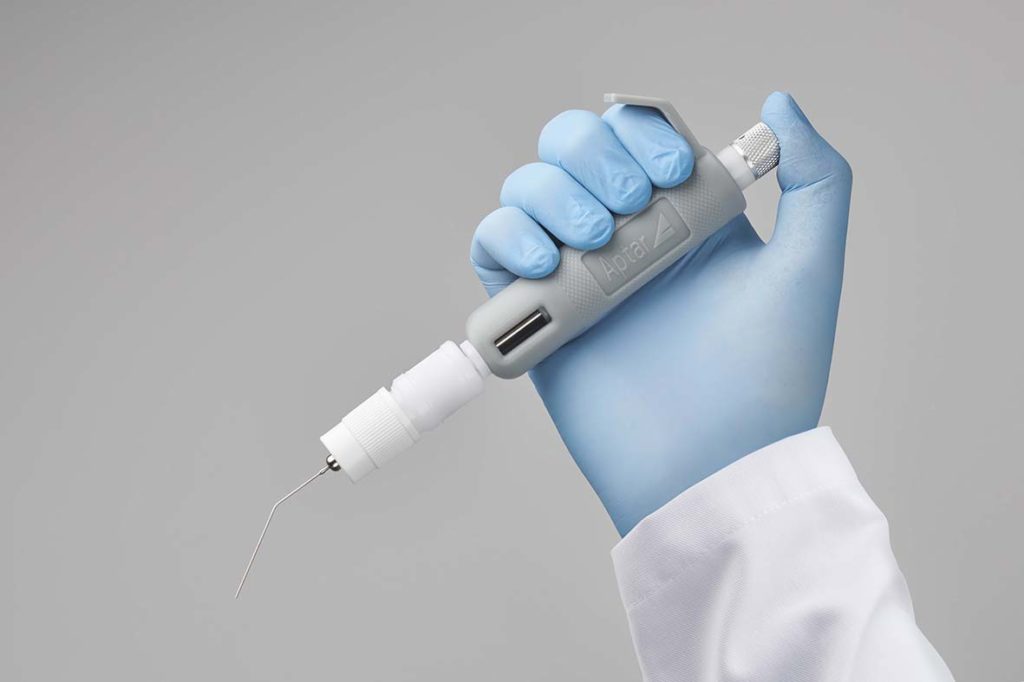
Lack of availability of a solution for administering inhaled powders to animals in preclinical respiratory research impedes rapid progress. This case study describes a collaborative project between Aptar Pharma and the Unit of Pharmaceutics and Biopharmaceutics, Brussels to address this need. The resulting solution, the Powder Administration Device for Animals, or PADA, enables precise, rapid and efficient administration of a powder directly to the lungs of mice, thereby supporting the development of dry powder and metered dose inhalers. Prototypes of the product were developed and progressively refined in line with customer feedback to produce a successful commercial solution. Lightweight, convenient, and reusable the PADA is a valuable tool for preclinical respiratory research, for example for investigations of lung deposition behavior and PK/PD studies.
Subscribe to Email Updates
Submit your email address below and stay up to date with the latest industry insights.
*Required. For details about how your email address will be used, read our General Terms and Conditions of Use, Privacy and Cookies Policy.
Related Articles
Request Access
Requesting access to Case Study – Pre-Clinical Respiratory Research with PADA.
How Can We Help?
