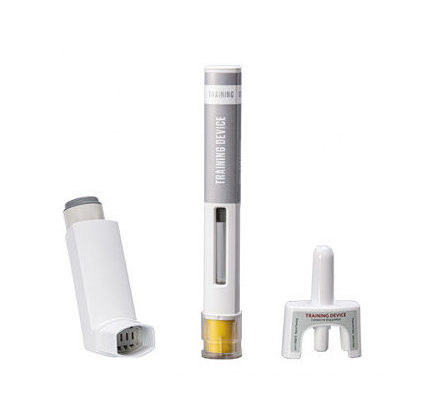
PADA – Starter Kit for Pre-Clinical Pulmonary Research in Mice
Preclinical Research Kits
Pharma
PADA (Powder Administration Device for Animals) is a unique starter kit from Aptar Pharma, designed to support pre-clinical pulmonary research in pharmaceutical, academic and governmental research laboratories.
PADA enables precise, rapid and efficient intra-tracheal administration of a powder directly to the lungs of mice. The kit contains a convenient, hand-held device with air-driven aerosol generator, ideal for use in early phase drug development of new pulmonary treatments such as asthma, COPD, lung cancers and cystic fibrosis.

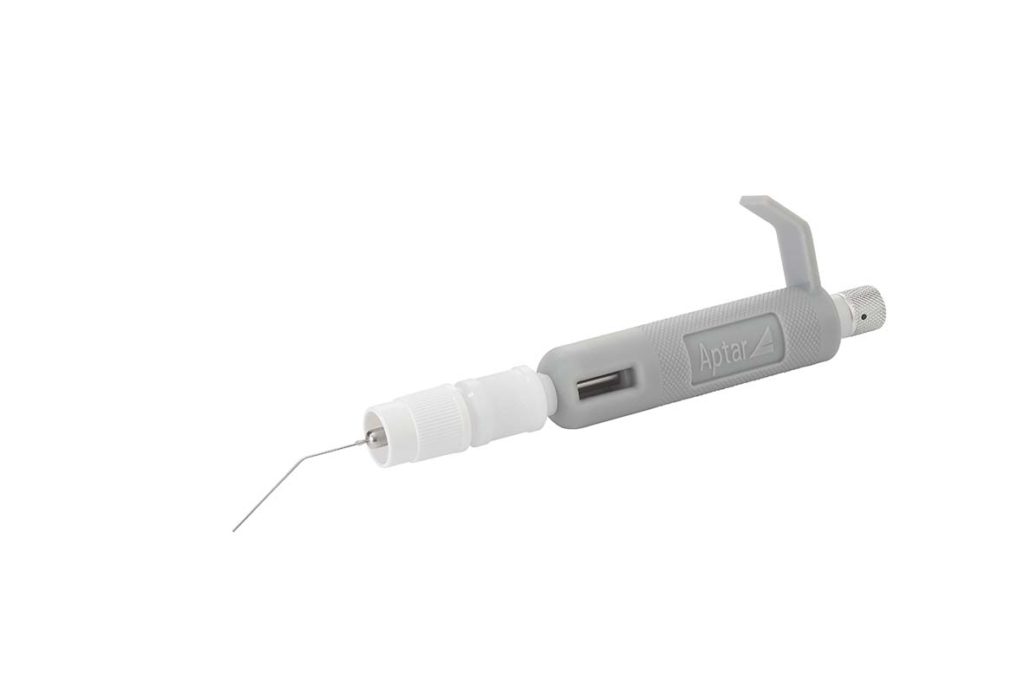




Answering an Unmet Need in Pre-Clinical Pulmonary Research
Ideal for pre-clinical pulmonary research
Our PADA starter kits offer everything you need for pre-clinical testing of pulmonary powder formulations in mice. Designed and developed for early phase drug development and trusted by both academics and researchers, our PADA kit contains all you need to begin your non-clinical work.
Precise and targeted powder administration to the lungs
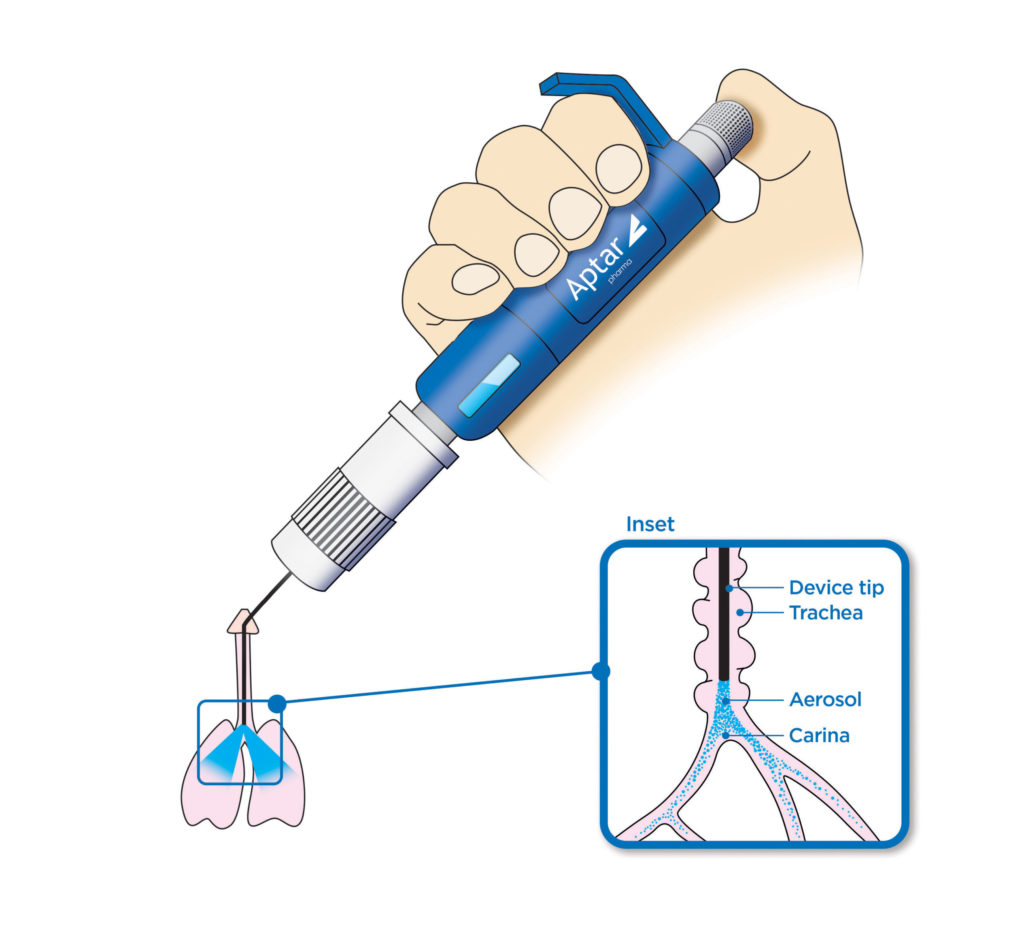
Aptar Pharma’s PADA kit enables precise testing, development and screening of respiratory drugs for both DPI and MDI applications, spray-dried and biologic dry powder formulations and their excipients, evaluation of inhalation and deposition in the lung, aerodynamic properties, PK/PD, and more. The powder is delivered via the trachea, directly to the lungs.
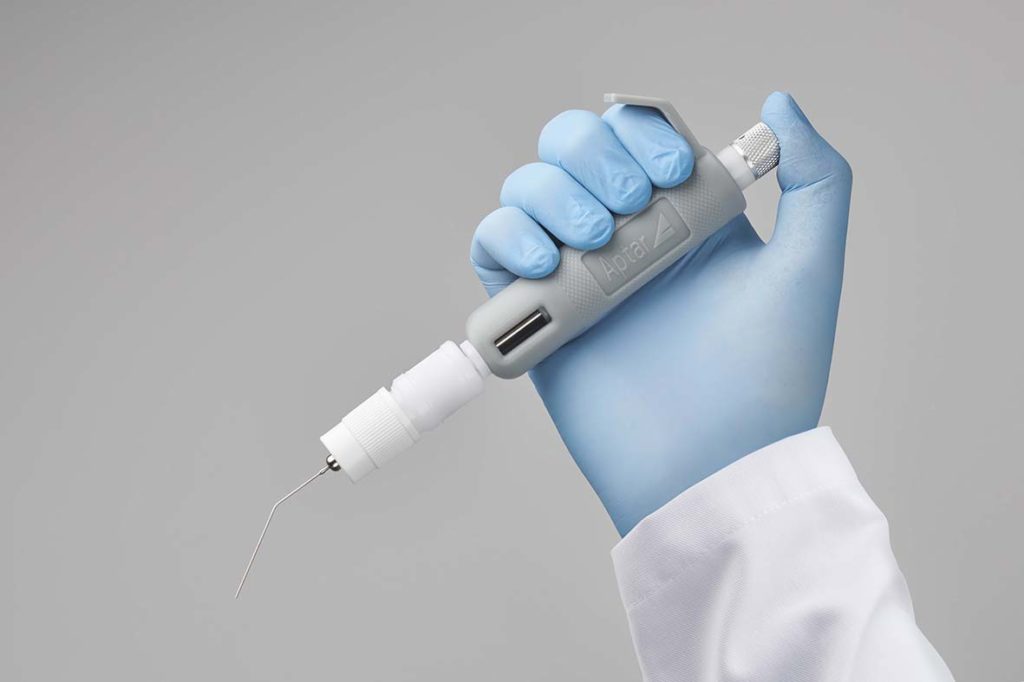
Ready-to-use system
Our PADA starter kit is a ready-to-use system from a global leader in pulmonary drug delivery devices. With decades of experience and scientific knowledge in respiratory solutions, Aptar Pharma delivers innovative and effective pulmonary solutions for both your pre-clinical and clinical work.
Satisfies an unmet pre-clinical research need
The PADA starter kit satisfies a real need in pre-clinical research in mice. Our kit is available to researchers and academics all around the world to develop innovative new vaccines and treatments, as well as more clinically-relevant models of pulmonary and systemic disease, lung imaging and aerosol drug delivery.
Supported by a comprehensive range of services
For your entire project development, benefit from Aptar Pharma’s broad range of services to accelerate and de-risk your new product development journey. These services include end-to-end support from R&D, regulatory support and guidance, regulatory submission as well as post launch support.

Aptar Pharma’s PADA Starter Kit Advantages
- Reliable, Targeted Delivery
- Convenient and Intuitive
- Trusted Pulmonary Expertise
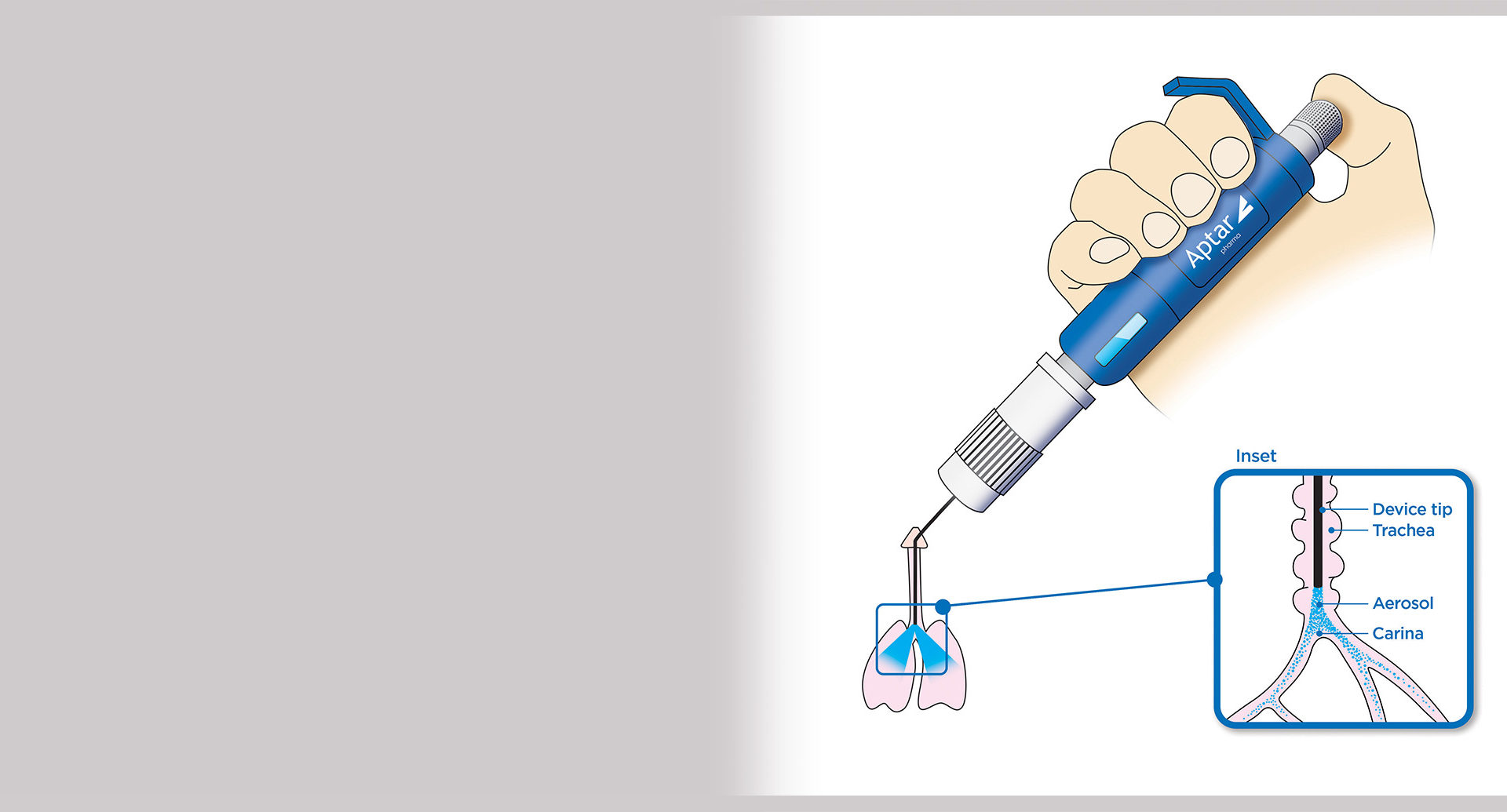
Allows precise testing of pulmonary drugs
PADA enables precise testing, development and screening of respiratory drugs for both DPI and MDI applications, spray-dried and biologic dry powder formulations and their excipients, evaluation of inhalation and deposition in the lung, aerodynamic properties, PK/PD, and more. The powder is delivered directly to the lungs via the trachea.
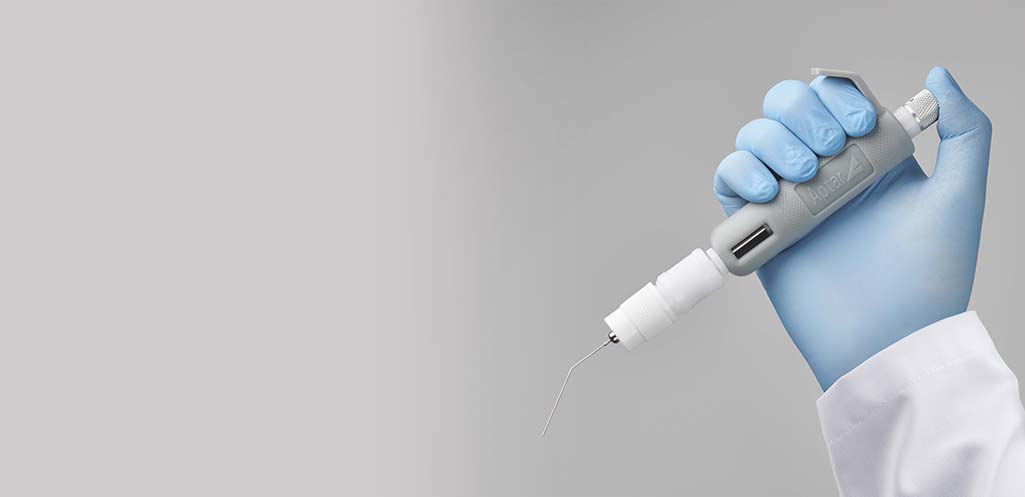
Combining convenience with ease-of-use
The PADA starter kit is easy and intuitive to use.

Built from our unrivalled expertise in Pulmonary drug delivery
As world leaders in asthma and COPD drug delivery devices and services, Aptar Pharma is uniquely positioned to support your early development work. Together with our pre-clinical pharmacological PK and PD disease models, our extensive inhalation facilities, team of experts and medical device design and development functions, we can accelerate and de-risk your next respiratory project.
Partner with Aptar Pharma in your Next Preclinical Pulmonary Research Project
To leverage our expertise in pulmonary drug delivery and get to market faster, why not speak to one of our experts today?
We Have a Broad Range of Value Added Services to Accelerate and Derisk Your Drug Product Development.
We Offer World-Leading Support Services for You at Every Stage of Your Product Development
Explore How We Serve Your Market
Request More Information
Requesting information on PADA – Starter Kit for Pre-Clinical Pulmonary Research in Mice.
How Can We Help?