
Advanced Parenteral Closure Solutions

As the injectable pharmaceutical market shifts toward more complex and sensitive drugs, Aptar Pharma has developed advanced parenteral closure solutions to derisk injectable drug development.
- PremiumFill® – vial stoppers and syringe plungers offer enhanced specifications to support compliance with the EMA GMP Annex 1 revision (2022) requirements and improve your operational efficiency.
- Ready-to-Use (RTU) – our gamma sterilization process guarantees the sterility of our parenteral closures at the time of use and helps reduce your operational costs.
- Rapid Transfer Port (RTP) bags – an essential element of EMA GMP Annex-1 compliant aseptic filling processes that can minimize the risk of injectable drug product contamination on sterile fill-finish lines.

Injectables
Injectables
Delivery Routes
-
Delivery Routes
- Eye Care
- Dermal
- Other Routes

Nasal Drug Delivery
Nasal Drug Delivery

Inhalation Drug Delivery
Inhalation Drug Delivery
-
Inhalation Drug Delivery
- Inhalation Drug Delivery Overview

Injectables
Injectables

Oral Liquid Dispensing
Oral Liquid Dispensing
-
Oral Liquid Dispensing
- Oral Liquid Dispensing
- Liquid Nutritional Supplements
- Oral Liquid Delivery Frequently Asked Questions
Unlock the Potential of your Injectable Primary Packaging
Advanced parenteral closure solutions to comply with a changing market
The pharmaceutical industry is evolving towards more complex and sophisticated drugs such as biologics and next-generation vaccines. These innovations offer new possibilities for treating and preventing various diseases but may involve more complex manufacturing processes and substantial research and development investments.
Alongside this progression, regulatory expectations are also changing, with agencies imposing stricter standards for safety, efficacy, and quality.
Leveraging decades of primary packaging expertise, Aptar Pharma can help our pharmaceutical partners navigate these increasingly complex landscapes. We have developed technologies and processes that can be combined to help you bring safer and more effective therapeutics to patients, reducing the overall risk, while improving your operation and accelerating time-to-market.

- PremiumFill®
- Ready-to-Use (RTU) Gamma Sterilized Parenteral Components
- Rapid Transfer Portbags (RTP)
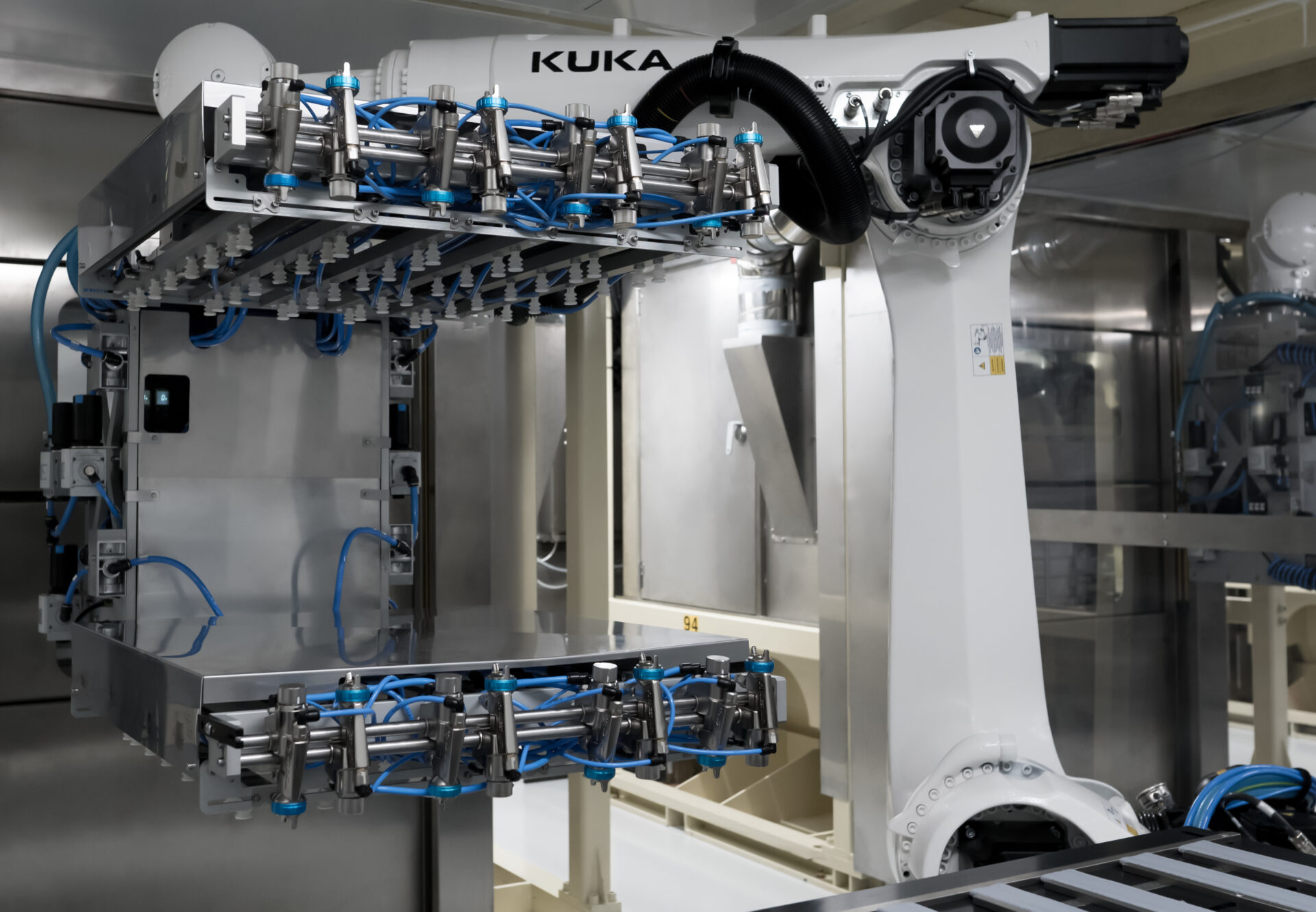
Improved specification for reduced contamination and improved operational efficiency
Our PremiumFill® vial stoppers and syringe plungers are processed in ISO-classified clean rooms all the way from molding through to final packaging, using state-of-the-art robotization. The enhanced particulate specifications of our PremiumFill® line can help optimize pharmaceutical operations, reduce the risk of contamination, and minimize reject rates, all while meeting the strict requirements of the 2022 EMA GMP Annex 1 revision.

Guaranteed sterility at the time of use to ensure patient safety and reduce operational costs.
Our RTU vial stopper and pre-filled syringe (PFS) formulations can be sterilized via a validated gamma sterilization process, which guarantees sterility at the point of use. Our RTU components help you to comply with EU GMP Annex 1 revisions regarding sterility assurance. Additionally, our RTU solutions eliminate the risk of introducing Tyvek particles on your filling line because they use PE-only secondary packaging. Optimize your operations with Aptar Pharma RTU sterility guaranteed components.

Reduce the risk of external contamination on drug product fill-finish lines
Our Ready-to-Sterilize (RTS) and Ready-To-Use (RTU) vial stoppers and syringe plungers can be supplied in a wide variety of Rapid Transfer Port bags. Connect your Rapid Transport Port bags directly to your isolator/RABS in order to prevent accidental contamination of your drug product with contaminants during your aseptic fill-finish process. Using isolators and port bags helps sterile manufacturers to comply with the 2022 EU GMP Annex 1 revision.
A premium approach to ensuring drug integrity and patient safety
As primary packaging is in direct contact with the drug product, its cleanliness is of prime importance. Contamination, whether in the form of fibers, particles or micro-organisms can pose a serious threat to patient safety. Pharmaceutical manufacturers must ensure that their manufacturing operates under the strictest controls from the earliest stages of their supply chain all the way through to the packaging of their finished product and final delivery to the patient.
Aptar Pharma’s advanced parenteral closure solutions have been developed to reduce the risk of contamination and to support the safe delivery of each dose to patients.
- Improved packaging cleanliness with PremiumFill® delivering improved specifications regarding key contamination criteria including levels of fibers, particulate, and biological matter.
- Sterility is guaranteed at the time of use with Aptar Pharma’s Ready-To-Use (RTU) vial stoppers and pre-filled syringe plungers.
- Prevent contamination of your drug product during fill-finish operations by choosing Rapid Transfer Port (RTP) bags for your advanced parenteral closures.

Shorten time to market with improved regulatory compliance
With the sky-rocketing costs of drug development programs and fierce product competition, any delay in bringing a drug to market can lead to significant financial losses. Pharmaceutical manufacturers are therefore looking to complete their drug development programs as quickly as possible, shortening their time to market.
Particulate contamination and sterility issues in parenteral pharmaceuticals can lead to adverse effects and are one of the main causes of regulatory recalls. The 2022 revision of the European Medicines Agency (EMA) Good Manufacturing Practice (GMP) Annex 1 illustrates the need for increased focus on these topics. Annex 1 now requires pharmaceutical manufacturers to implement a Pharmaceutical Quality System (QS) and demonstrate that they have developed a comprehensive Contamination Control Strategy (CSS) that includes all operations from their supply chain to their final product.
Aptar Pharma’s advanced parenteral closure solutions can be applied to help customers address these stringent requirements through:
- Tighter and improved specifications on key contamination criteria (fibers, particulate, biological matter) with PremiumFill® and providing an industrial means to help support your CCS.
- Meeting the sterility assurance requirements using our Ready-to-Use (RTU) gamma sterilized products and limit the risk of Tyvek-related fiber contamination.
- Compliance with EMA GMP Annex 1 revision requirements regarding the use of isolators/RABS that limit the risk of accidental contamination to support your Contamination Control Strategy (CSS) by using Rapid Transfer Port (RTP) bags.

Improved operational efficiency
With the rising costs associated with drug development and manufacturing, any operational inefficiency, product rejects or eventual product recalls can lead to significant financial losses for pharmaceutical companies. Selecting the right advanced parenteral packaging components can therefore help reduce these risks, ultimately mitigating the total cost of ownership for higher quality packaging and promoting the long term-success for your drug on the market.
- With their improved specifications, including particle contamination limits, PremiumFill® vial stoppers and pre-filled syringe plungers can help minimize rejection rates on drug product fill-finish lines, reduce scrap rates and lower the overall risk of recalls.
- Ready-to-Use (RTU) syringe plunger and vial stopper solutions guarantee sterility at the time of use and eliminates the need for a drug manufacturer to acquire its own costly sterilization equipment and validate their own sterilization process, thereby saving them time, production space and costs.
- Minimize the risk of accidentally introducing particles to products on your sterile filling line, reducing product rejects and the risk of product recalls.

Product Details
Contact Aptar Pharma’s Parenteral Expert Team Today
Learn more about how we can support your compliance with EMA GMP Annex 1 revisions and optimize your operations with our advanced injectable packaging solutions.
This Might Also Be of Interest

GLP-1 Drug Development: Leveraging Integrated Solutions to Improve Patient Engagement
Webinars, Pharmaceutical, Innovation & Insights, Drug Delivery Innovations, Market Insights, Product Solutions

USP <382>: A Shift in Injectable Packaging Requirements
Publications, Pharmaceutical, Innovation & Insights, Market Insights, Product Solutions

EU GMP Annex 1: New Standards for Injectable Drug Manufacturing
Webinars, Pharmaceutical, Innovation & Insights, Drug Delivery Innovations, Market Insights, Product Solutions

Optimizing Autoinjector Performance for Drug Development
Webinars, Pharmaceutical, Product Solutions, Innovation & Insights, Market Insights
