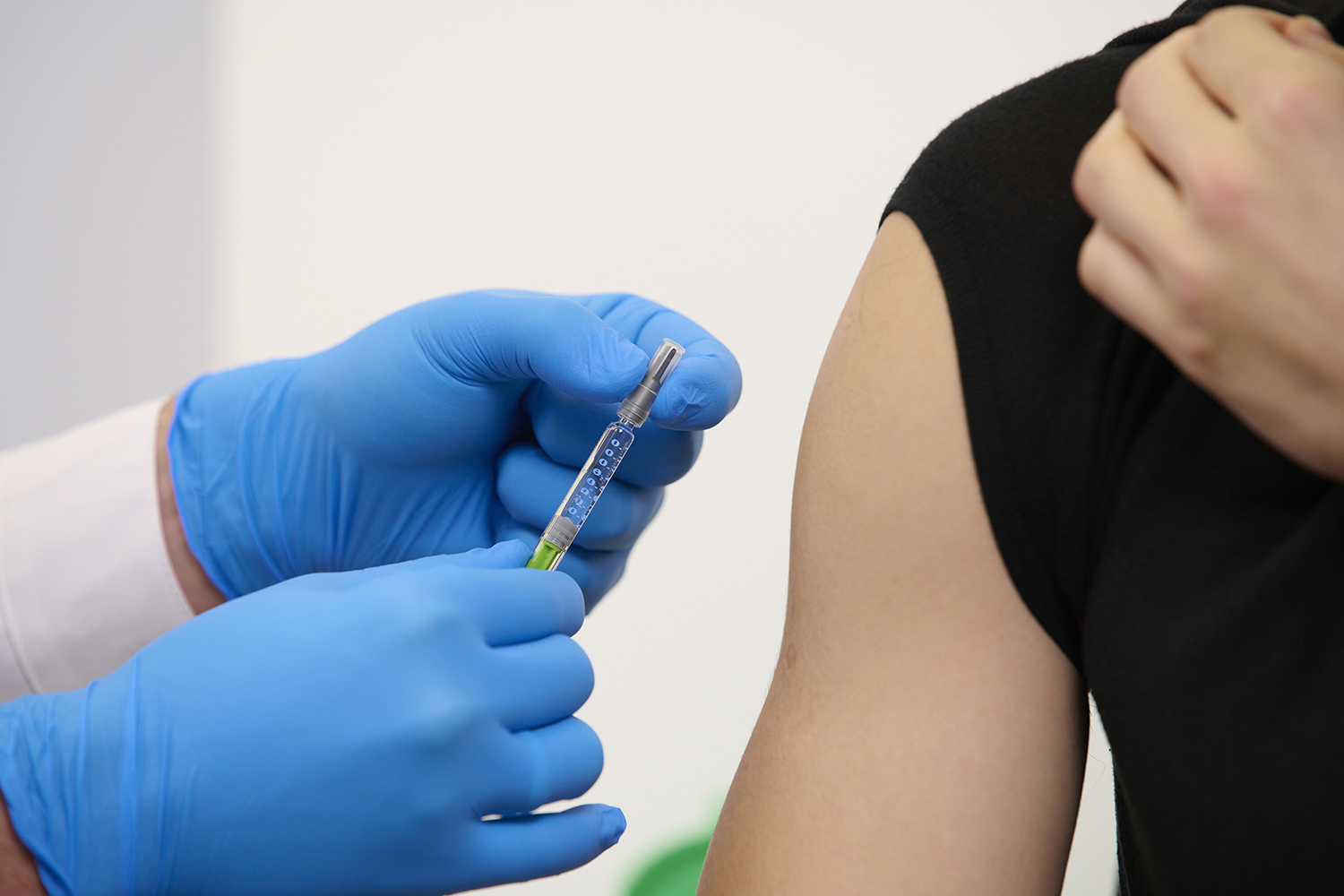
Injectables Closure Excellence: De-risking Your Drug Development

Aptar Pharma is a world-leading supplier of injectable packaging components. Whether you are developing sensitive biologics, vaccines, small molecules or generics, our vial stoppers, pre-filled syringe closure components and advanced parenteral closure solutions can accelerate and derisk your project, while striving to ensure patient safety.
We are shaping the future of injectables, together with our Pharma partners.

Injectables
Injectables
Delivery Routes
-
Delivery Routes
- Eye Care
- Dermal
- Other Routes

Nasal Drug Delivery
Nasal Drug Delivery

Inhalation Drug Delivery
Inhalation Drug Delivery
-
Inhalation Drug Delivery
- Inhalation Drug Delivery Overview

Injectables
Injectables

Oral Liquid Dispensing
Oral Liquid Dispensing
-
Oral Liquid Dispensing
- Oral Liquid Dispensing
- Liquid Nutritional Supplements
- Oral Liquid Delivery Frequently Asked Questions
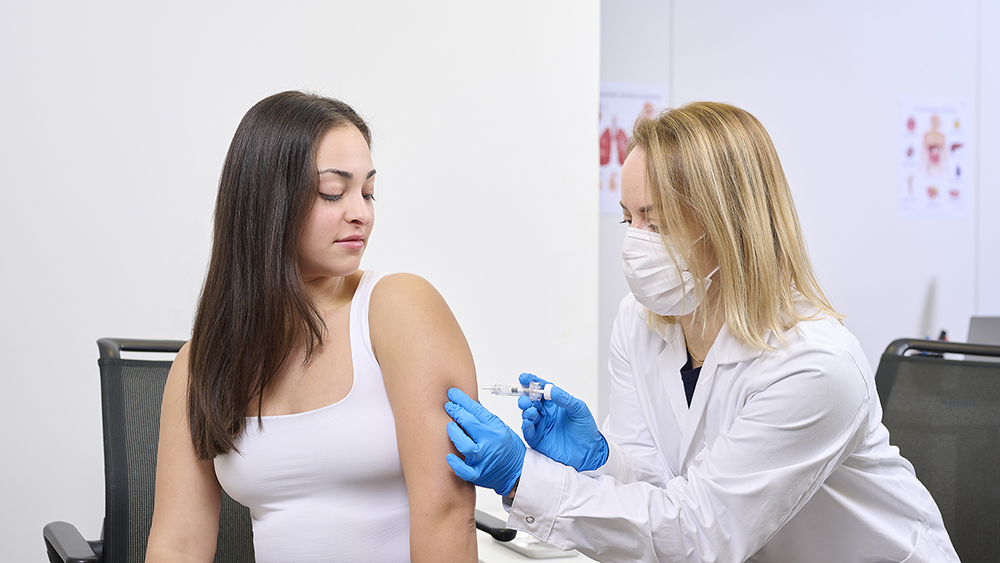
How Can A Partner Enable Your Injectables Development?
The pharmaceutical ecosystem is evolving rapidly
For over 50 years, Aptar Pharma has been supporting the development of injectable drugs with pre-filled syringes, vials, lyophilization and cartridge components. Leveraging this experience in rubber formulation together with world-class manufacturing processes and analytical services, we partner with your drug development teams to help you select the best injectable rubber closure for your needs and get you to market in no time.

PremiumCoat® Platform
Protecting your most sensitive injectable drugs
ETFE film-coated vial stoppers, syringe plungers and services to accelerate your parenteral drug development. Protect your most sensitive injectable drugs and your patients with our pure bromobutyl formulation combined with our the market coating technology and state-of-the-art process
Find out more about PremiumCoat®ETFE film-coated vial stoppers and syringe plungers
Vial Containment Solutions
Complete range of vial stoppers for liquid and lyophilized parenteral drugs
Our Market proven elastomeric formulations pure Bromobutyl and market proven Chlorobutyl combine with proprietary designs to enable efficiency on your filling line and lyophilization processes.
Pre-Filled Syringe (PFS) Solutions
One-stop-shop for your PFS closures
A world-leading range of rigid needle shields, syringe plungers and tip closures are available in a variety of format and formulations for all your pre-filled applications.
Advanced Parenteral Closure Solutions
Derisk your development with PremiumFill®, RTU Gamma products & RTP bags
A complete range of solutions that combines with your closure component to optimize operations, meet regulatory requirements and derisk drug development. PremiumFill® offers improved specification on contamination criteria, Ready-to-Use (RTU) gamma sterilization guarantees sterility at the time of use and Rapid Transfer Port bags minimize the risks of contamination on your aseptic lines.
Parenteral Analytical and Development Services
World-class expertise in packaging validation and development
Accelerate and derisk your development by partnering with. our parenteral packaging experts. We offer testing for extractables and leachables, particulate analysis, and Container Closure Integrity (CCIT) with Gateway Analytical. At Aptar Pharma, we also offer injectable packaging customization and validation for your specific requirements.
We’re Transforming Your Expectation Of What An Injectables Partner Can Be
Increasing capacity to ensure long-term security of supply
Aptar Pharma has successfully launched its large-scale, $180 million expansion program. Initiated in 2020, this multisite international endeavor is set to significantly enhance production capacity across all product lines, enabling the delivery of over 10 billion injectable components annually once completed.
The expansion program already delivered a new extension to the existing factory in Granville, France with a focus on PremiumCoat® and PremiumFill® manufacturing. A new extension is also being built in Congers, USA and is bringing product manufacturing on the US soil for the first time. The acquisition of Hengyu in Weihai, China, also brings extra capacity as well new products dedicated to the Asian market.
The crown jewel of this expansion program is the new, state-of-the-art manufacturing plant being built in Granville, France, meters away from our original manufacturing plant. This new facility is already housing a brand-new rubber mixing line that will feed extra manufacturing lines dedicated to PremiumCoat® and rigid needle shields manufacturing. With the construction already completed, our new factory will deliver its first commercial batch as early as March 2024.

Expanding capabilities to enable safety & efficacy for your drugs and your operations
Our expansion is attuned to the needs of the market regarding increased quality. In addition to the extra capacity that we are building, we are investing in state-of-the-art technologies to help you meet the rising regulatory expectations of the market and enable the safe delivery of millions of doses to your patients worldwide.
Particulate cleanliness and contamination control are at the heart of our expansion program. With the recent implementation of Annex 1 revision of Good Manufacturing Practices (GMP) of the European Medicines Agency (EMA), sterile drug manufacturers must be able to demonstrate the implementation of a Contamination Control strategy (CCS), which encompasses their whole manufacturing process from suppliers to the finished products. At Aptar Pharma, we are expanding our cleanroom manufacturing capabilities to ensure the cleanliness of our injectable closure components.
We are also developing automation at all from the earliest stages of rubber mixing, to components molding and final washing, We’re using state-of-the-art robotization and digitalization to improve reproducibility and traceability while mitigating human-related contamination.
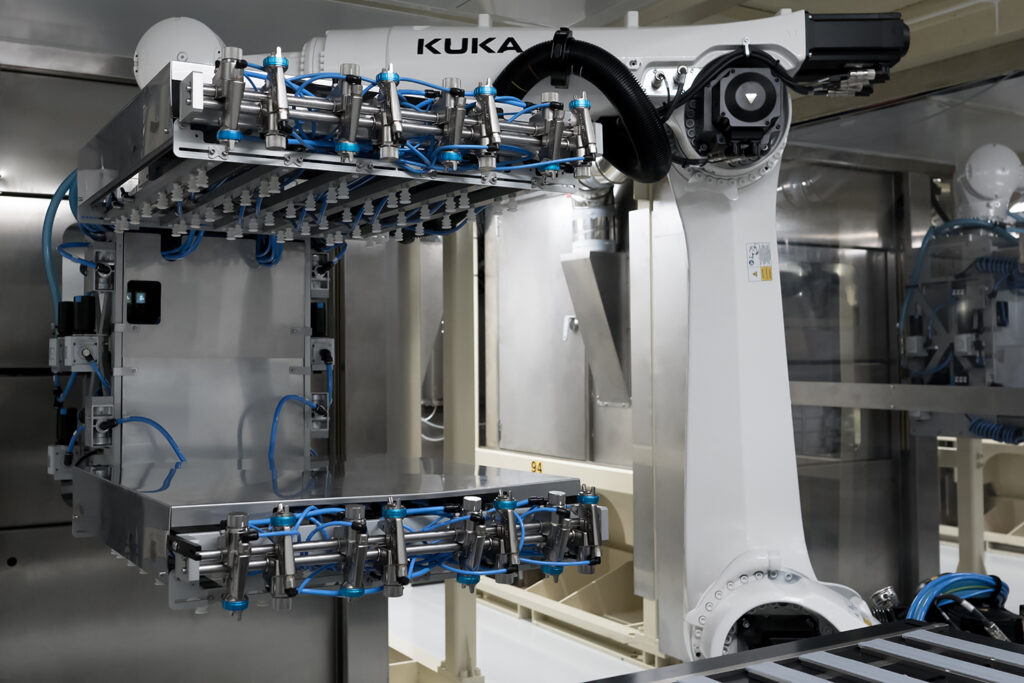
We are investing, partnering with you and striving for your success
At Aptar Pharma, we believe that partnership is at the heart of every success. This is why we have been dedicated, for over 50 years, to working closely with our customers to ensure that your needs are being addressed efficiently and rapidly. Our experts will work with your teams to ensure that our solutions meet your expectations.
In a time where the market is undergoing major changes, where security of supply and flexibility have become essential, we have developed our industrial tools to be adaptable to our customers’ supply needs. In addition to having designed our new factory with modularity in mind and allowing for rapid reorganization of our production mix, we are ready to further extend our production capacity for our PremiumCoat®, PremiumFill® and rigid needle shield products.

Sustainability at the heart of our transformation
With our new factory in Granville, France, we took our sustainability initiative to the next level by achieving LEED silver certification and by being part of the water stewardship program. We have also optimized our utilities for energy efficiency and installed solar panels for on-site energy production.
Robotization is also one of the many ways Aptar Pharma promotes wellbeing and safety at work by diminishing physical strains on our colleagues and risks of accidents. These transformations also help promote equity at work, in line with Aptar’s sustainability commitment.
Learn more about how sustainability is at the heart of Aptar Pharma’s expansion

Development Services For Accelerating and Derisking Your Drug Development
Aptar Pharma supports your drug development beyond parenteral packaging components
Because we understand that our customers’ success goes beyond the supply of closure components, Aptar Pharma has dedicated the past decade to building up our service capabilities. In addition to developing customized injectables solutions for our customer’s most specific requirements, we are leveraging the expertise of Gateway Analytical to provide a wide range of parenteral analytical services to help our customers select, validate or troubleshoot your packaging. These services include particle counting, sizing and identification, Container Closure Integrity Testing (CCIT) and extractable & leachable studies.

By working with Noble, our customers can also go beyond drug development to focus on our patients’ experience and ensuring they adhere to their treatment. To that end, Noble offers human factor studies and develops training devices to help onboard patients and ensure your delivery system answer their needs.
To further promote long-term treatment adherence and enhance patient experience, Aptar Digital Health offers innovative digital solutions that support every step of the patient journey. From connected devices to digital patient support programs, our solutions can round-up your product benefits, help you differentiate from competition and improve the lives of millions of patients worldwide.
Injectable Product Solutions
We have been setting the standards in injectable drug delivery for decades, offering a wide range of high-quality, market-leading components for all your injectable challenges. We partner with major pharmaceutical and biotechnology companies, and offer a wide range of injectable components as well as support services, including analytical testing, regulatory support and post launch support.
Search Products
Start a Project With Us
We Thrive on Transforming Ideas into Opportunities – Let Yours be Next.
Recently Published Resources
Aptar Pharma talks training solutions and education for self-injection
Publications, Pharmaceutical, Innovation & Insights, Drug Delivery Innovations, Market Insights, Product Solutions

How High Quality Film and Coated Elastomeric Stoppers Can Accelerate and Derisk your ...
Webinars, Pharmaceutical, Market Insights, Product Solutions, Innovation & Insights
Applying Connectivity to Enhance The Training Standard of Care for Self-Injection
Publications, Pharmaceutical, Product Solutions, Innovation & Insights, Drug Delivery Innovations, Brand Differentiation

Aptar Pharma expert Q&A on digital health and connected drug delivery
Publications, Pharmaceutical, Innovation & Insights, Drug Delivery Innovations, Brand Differentiation, Product Solutions
How Can We Help?
- Explore Our Latest Innovation & Insights
- Discover Our Product Solutions
- Find Resources
- See What's New in Sustainability
-
Partner with Us
We’re here to help with product development. Whether developing a new device, looking to become a supplier—or everything in between—your partnership is valued.
